Noble Metal-Organic Framework Green Catalysis – Scientific and Technological Innovation Helps Peak Emissions, Carbon Neutrality
Catalyst is widely used in chemical, petrochemical, environmental protection, and other fields, which can accelerate the reaction rate, shorten the reaction time, reduce the energy consumption, and improve production efficiency. Advanced catalysts for industrial process optimization/enhancement are an effective way to achieve energy saving and emission reduction and help peak emissions and carbon neutral. Among them, heterogeneous catalysis is a greener low-carbon technology, and its core target lies in the development of high activity, stable structure, and reusable catalysts. In the context of carbon neutrality, the development of efficient and stable advanced catalyst materials is of great significance not only for energy conservation and emission reduction in the traditional chemical industry but also for CO2 conversion with high added value in carbon capture, utilization, and storage (CCUS) technology.
Palladium catalysts have been widely studied and applied for their excellent catalytic activity and efficiency in various reaction systems. By uniformly dispersing noble metal atoms on the surface of the carrier to form heterogeneous catalysts, the catalytic efficiency can be further improved, and it is easy to recycle the catalyst, saving cost and energy consumption. Metal-organic framework (MOF) materials are crystalline porous materials composed of metal ions/clusters and organic ligands connected by coordination bonds. As an ideal heterogeneous catalytic platform, MOF not only has high density and uniform distribution of monatomic metal active sites, but also its high porosity is conducive to mass transfer, and the pore structure of a specific size can also achieve shape selection catalysis. However, due to the inertness of the coordination bonds between Pd(II) and ligands and the fact that Pd(II) is easy to be reduced under conventional solvothermal conditions, the synthesis of Pd-MOF with precious metal Pd(II) as an inorganic node is limited and has not been broken through yet.
He Tao, a postdoctoral fellow jointly trained by the Key Laboratory of Advanced Functional Materials of the Ministry of Education and the Beijing Key Laboratory of Green Catalysis and Separation, and others have innovatively constructed the first stable mesoporous MOF based on Pd(II) multinuclear clusters through metal metathesis, and use it to photocatalytic CO2 reduction and another catalytic reactions, won the excellent catalytic effect (Figure 1). The results were published in J. Am. Chem. Soc. (DOI: 10.1021/ jacs.1c04077).
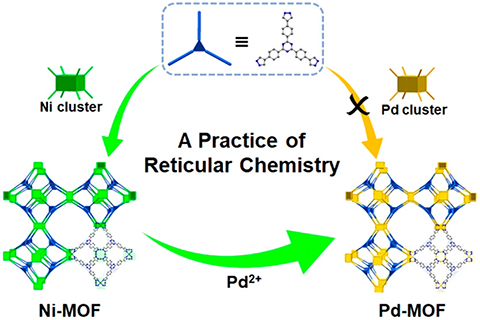
The stable and mesoporous Ni-MOF was obtained by kinetic control (BUT-33(Ni), the results have been published in J. Am. Chem. Soc., 2020, 142, 13491). In this work, Pd-MOF — BUT-33(Pd) was successfully obtained by using BUT-33 (Ni) as the template and deuterium as the medium to avoid the reduction of Pd(II) and retain the porous structure of the original MOF. BUT-33(Pd) has good chemical stability and mesoporous properties and abundant and uniformly dispersed Pd(II) active sites. Under light conditions, BUT-33(Pd) can catalyze the conversion of CO2 to CH4 (288 mol g−1h−1) and CO (269 mol g−1h−1). The photocatalytic performance of BUT-33(Pd) is better than those of most MOF catalysts under the same conditions. In addition, BUT-33(Pd) also showed good catalytic performances (> yield 90%) in classic organic cross-coupling reactions such as Suzuki and Heck.
This research provides a new idea for constructing efficient and stable monoatomic noble metal heterogeneous catalysts and provides new materials and technical support for green catalytic processes in the fields of CO2 conversion and the fine chemical industry. The new methods and materials developed in this work will promote the goals of energy saving and emission reduction in chemical catalytic process and CO2 resource utilization and are expected to help achieve the goal of carbon emissions peak and carbon neutrality as soon as possible.
